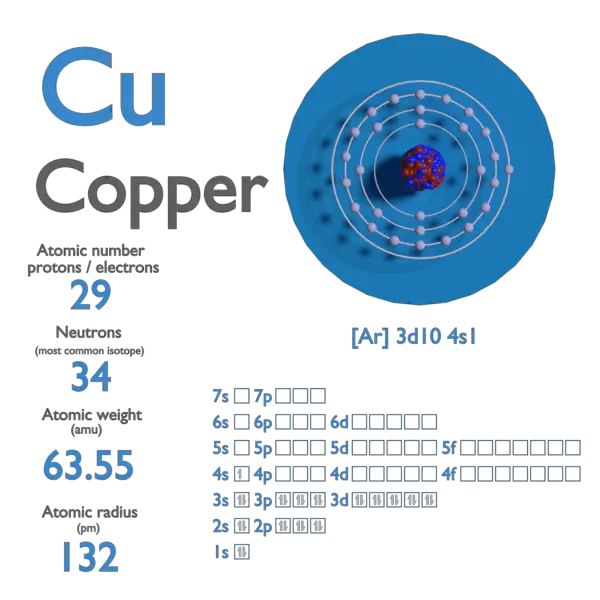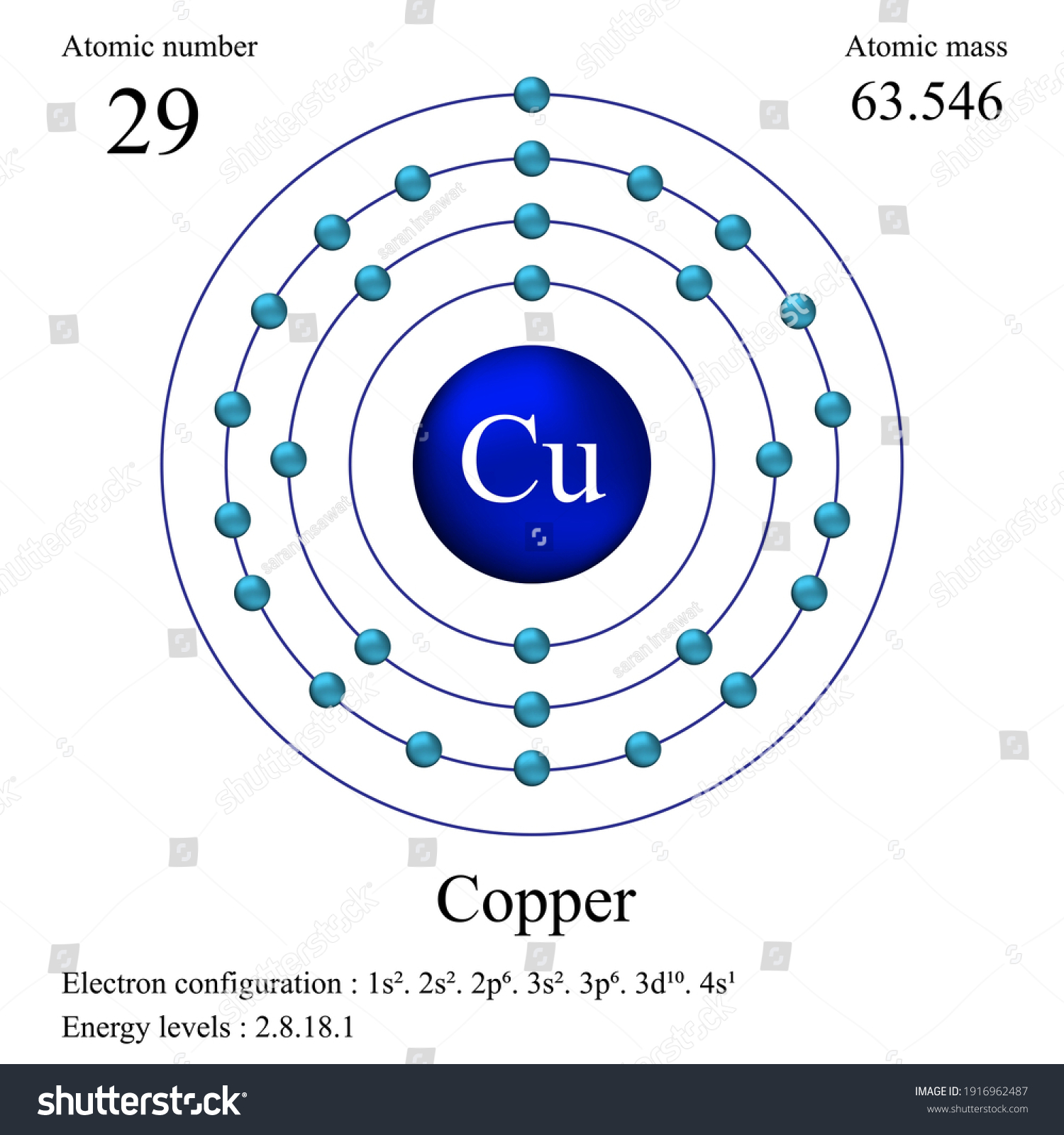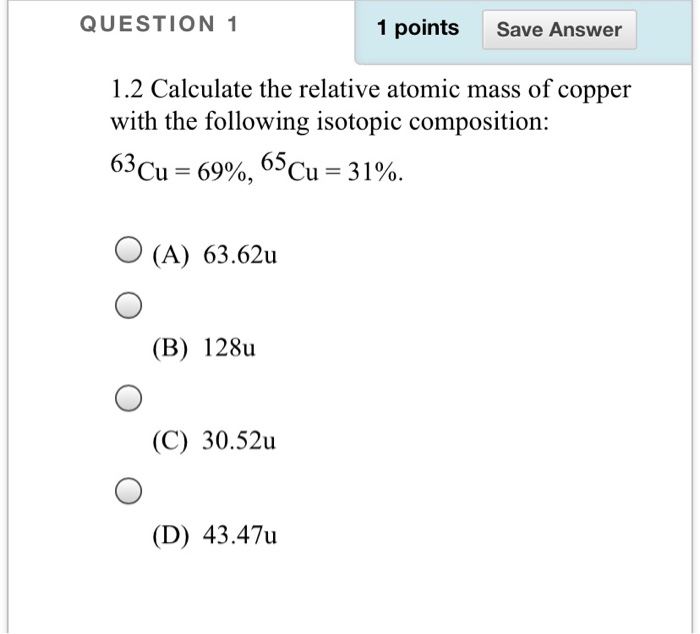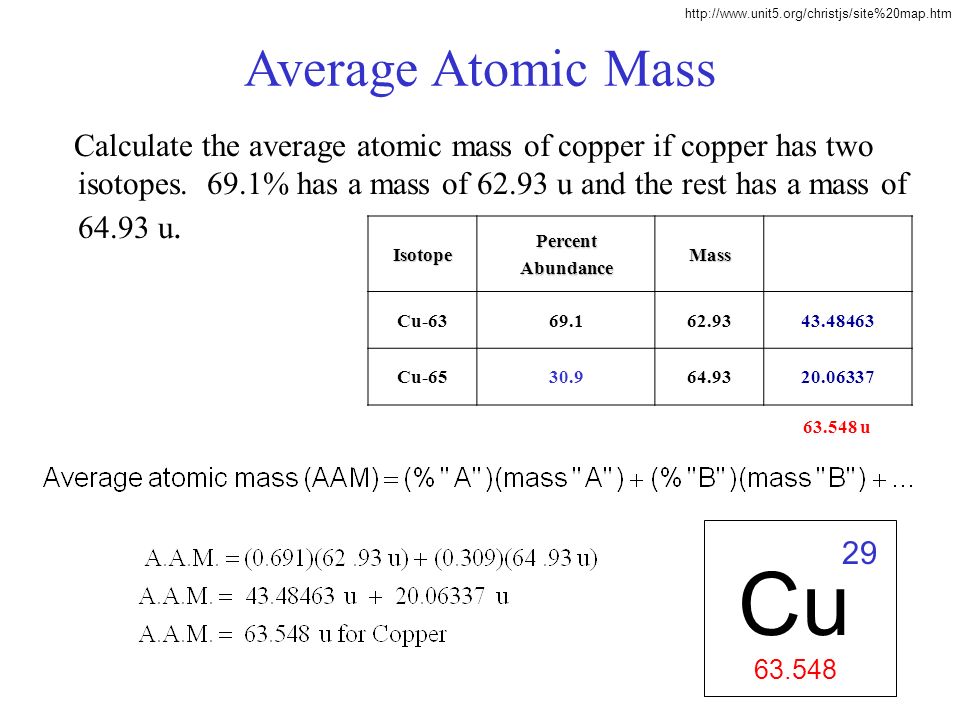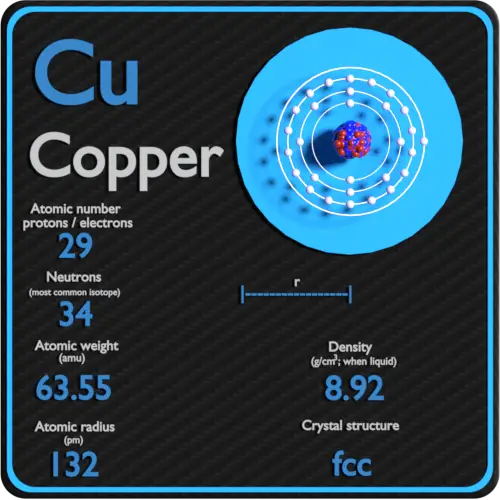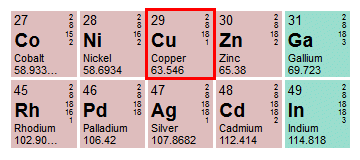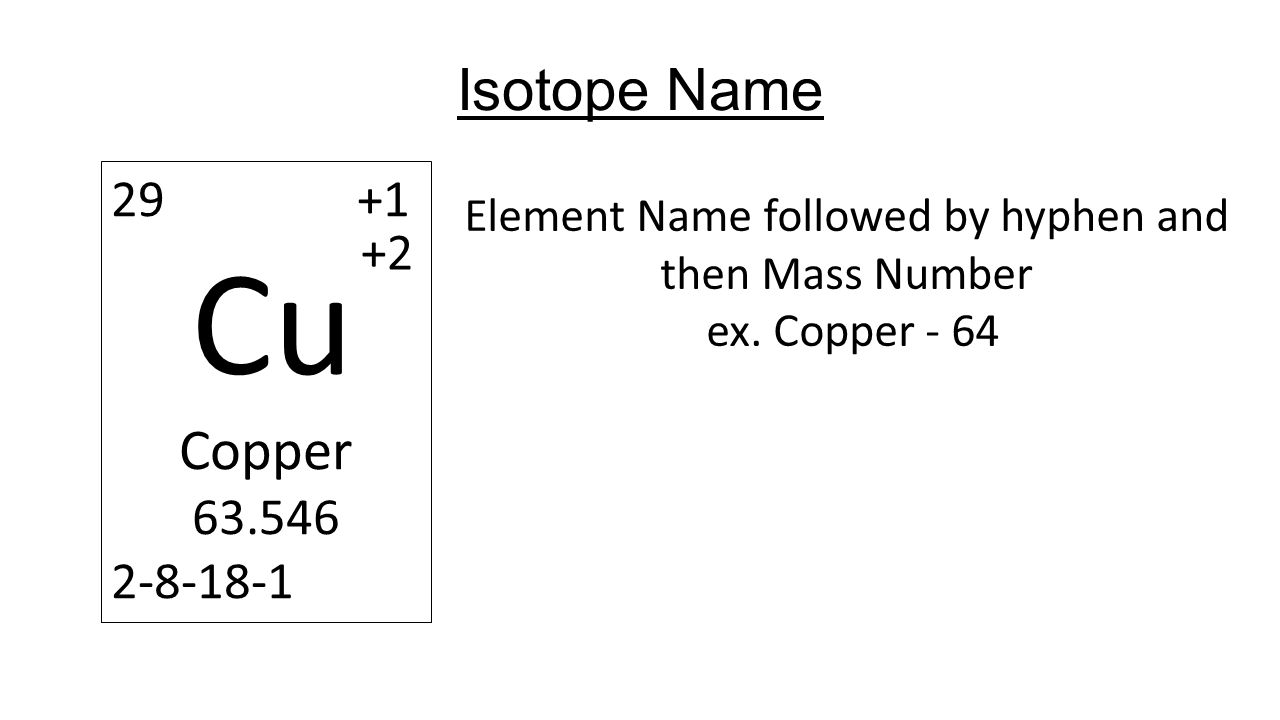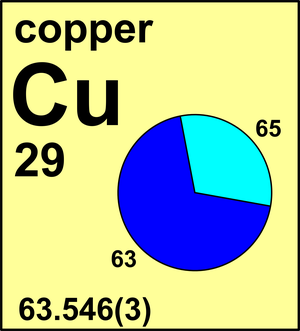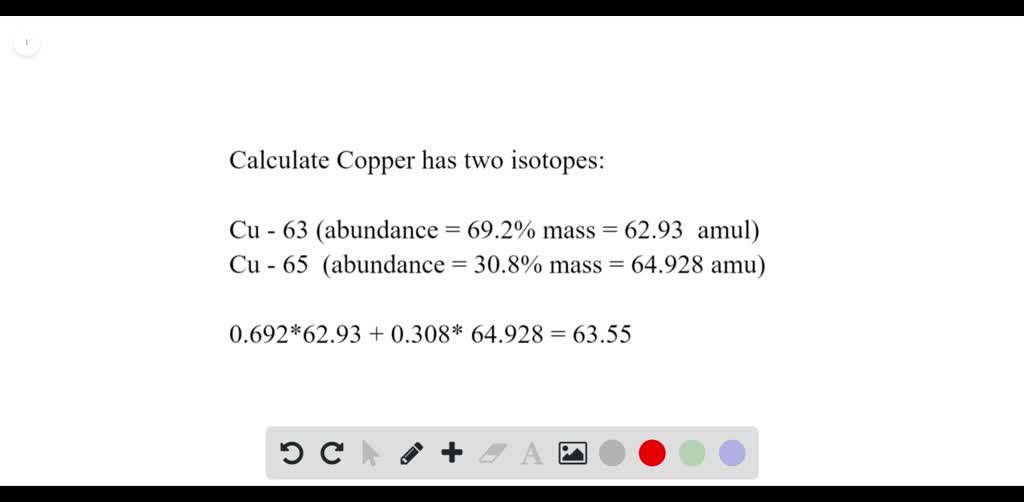
SOLVED:Calculate Copper has two isotopes: C u-63 (abundance =69.2 \% mass =62.930 amul and \mathrm{Cu}-65 (abundance =30.8 \%, mass =64.928 amu). Calculate the atomic mass of copper.

1 Average Atomic Mass Chemistry Notes. 2 Relative Atomic Mass Masses of atoms expressed in grams are very small, for example: One atom of Oxygen ppt download

An element has two isotopes with atomic masses (A - 1) and (A + 3) respectively. The average atomic mass is A . Calculate the mole percentage of the heavier isotope.

Copper consist of two isotopes Cu 63 and Cu 65 whose relative abundance is 69% and 31% respectively - Brainly.in

Defining how to calculate relative atomic mass of element relative isotopic mass definition gcse chemistry Calculations igcse O Level revision notes

Cu has only two naturally occurring isotopes, 63Cu and 65Cu . If the atomic mass of Cu is 63.546 , then the natural abundance of the ^63Cu isotope will be approximately :

Calculate mass of `Cu` in `3.67 xx 10^(3)g CuFeS_(2)` ? (Atomic mass `Cu = 63.5 Fe = 56,S = 32)` . - YouTube