PPT - Bioequivalence studies: Regulatory Requirements on Conduct & Documentation of BE. PowerPoint Presentation - ID:5123884
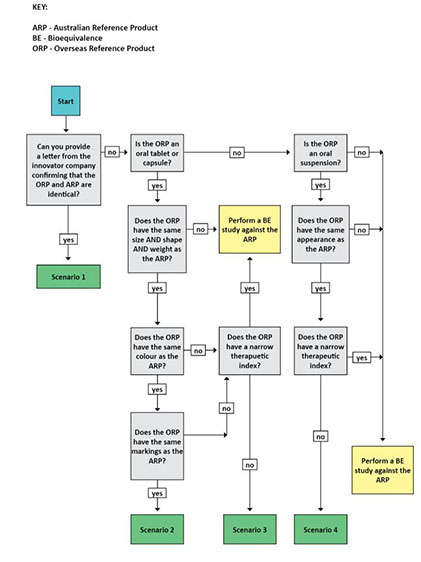
15.6 Choice of the reference product for bioequivalence of generic medicines | Therapeutic Goods Administration (TGA)
![PDF] The basic regulatory considerations and prospects for conducting bioavailability/bioequivalence (BA/BE) studies – an overview | Semantic Scholar PDF] The basic regulatory considerations and prospects for conducting bioavailability/bioequivalence (BA/BE) studies – an overview | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f7e13872d81da37ef0bef45811f8303c900f3753/2-Figure1-1.png)
PDF] The basic regulatory considerations and prospects for conducting bioavailability/bioequivalence (BA/BE) studies – an overview | Semantic Scholar

GENERIC DRUGS: Guidelines for bioequivalence studies: Vishwakarma, Pushpendra Kumar: 9783639343779: Amazon.com: Books

A pragmatic regulatory approach for complex generics through the U.S. FDA 505(j) or 505(b)(2) approval pathways - Klein - 2021 - Annals of the New York Academy of Sciences - Wiley Online Library
Provisional Translation (as of October 2021)* Administrative Notice September 14, 2021 To: Pharmaceutical Affairs Section, Prefe
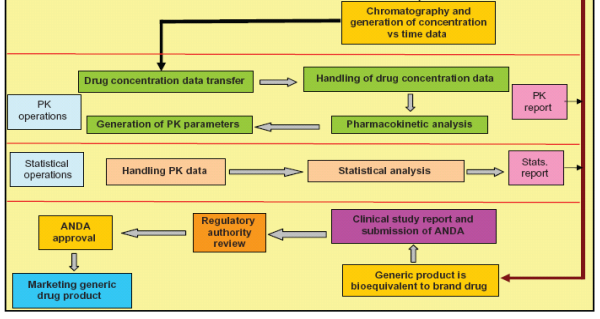
Study of regulatory requirements for the conduct of bioequivalence studies in US, Europe, Canada, India, ASEAN and SADC countrie

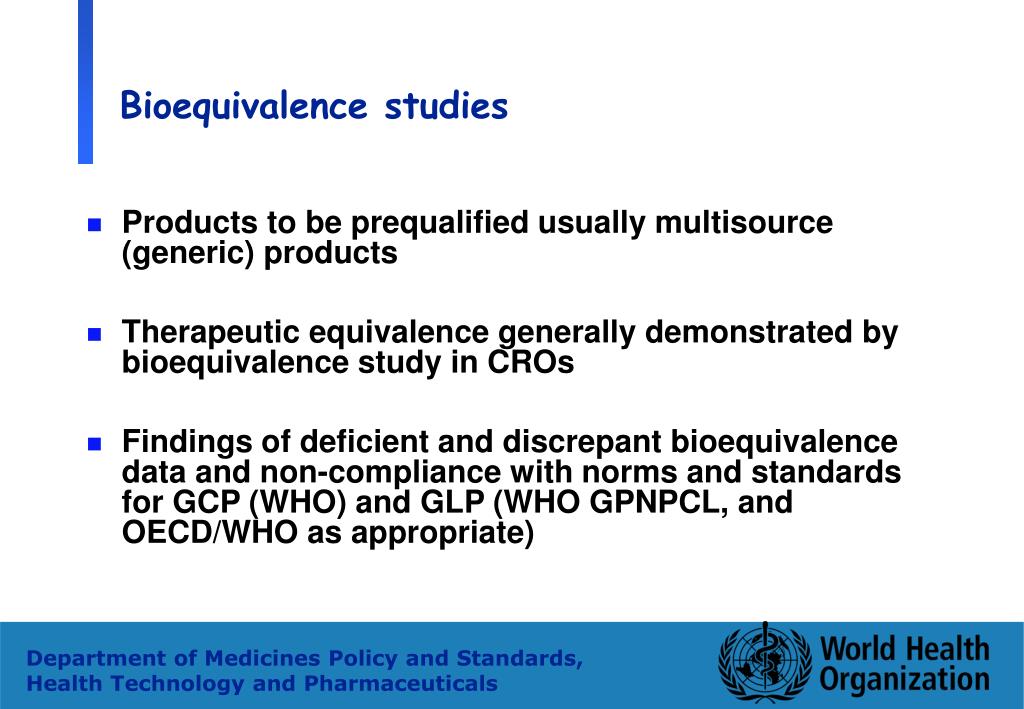
PDF) Implementation of Bioequivalence Studies for Approval of Generic Drug Products in Sudan: Current Status | abubakr Nur - Academia.edu

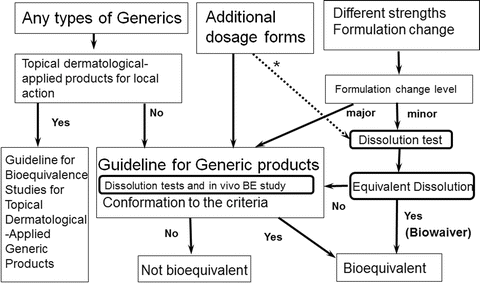
Bioequivalence of topical generic products. Part 2. Paving the way to a tailored regulatory system - ScienceDirect

Pharmacogenetic perspectives in improving pharmacokinetic profiles for efficient bioequivalence trials with highly variable drugs: a review | International Journal of Pharmacokinetics