A container of nitrogen (an ideal diatomic gas, molecular weight=28) is at a pressure of 2 atm and - Brainly.com

Four identical rods are joined end to end to form a square. The mass of each rod is M . The moment of inertia of the square about the median line is

Four identical rods are joined end to end to form a square. The mass of each rod is `M`. The mom... - YouTube

SOLVED: Question 3: Studiesl6 indicate that the earth's vegetative mass, or biomass for tropical woodlands, thought to be about 35 kilograms per square meter (kglm2 ), may in fact be too high
What is total square footage of space on the ISS? How many people can be accommodated on the ISS at one time? - Quora

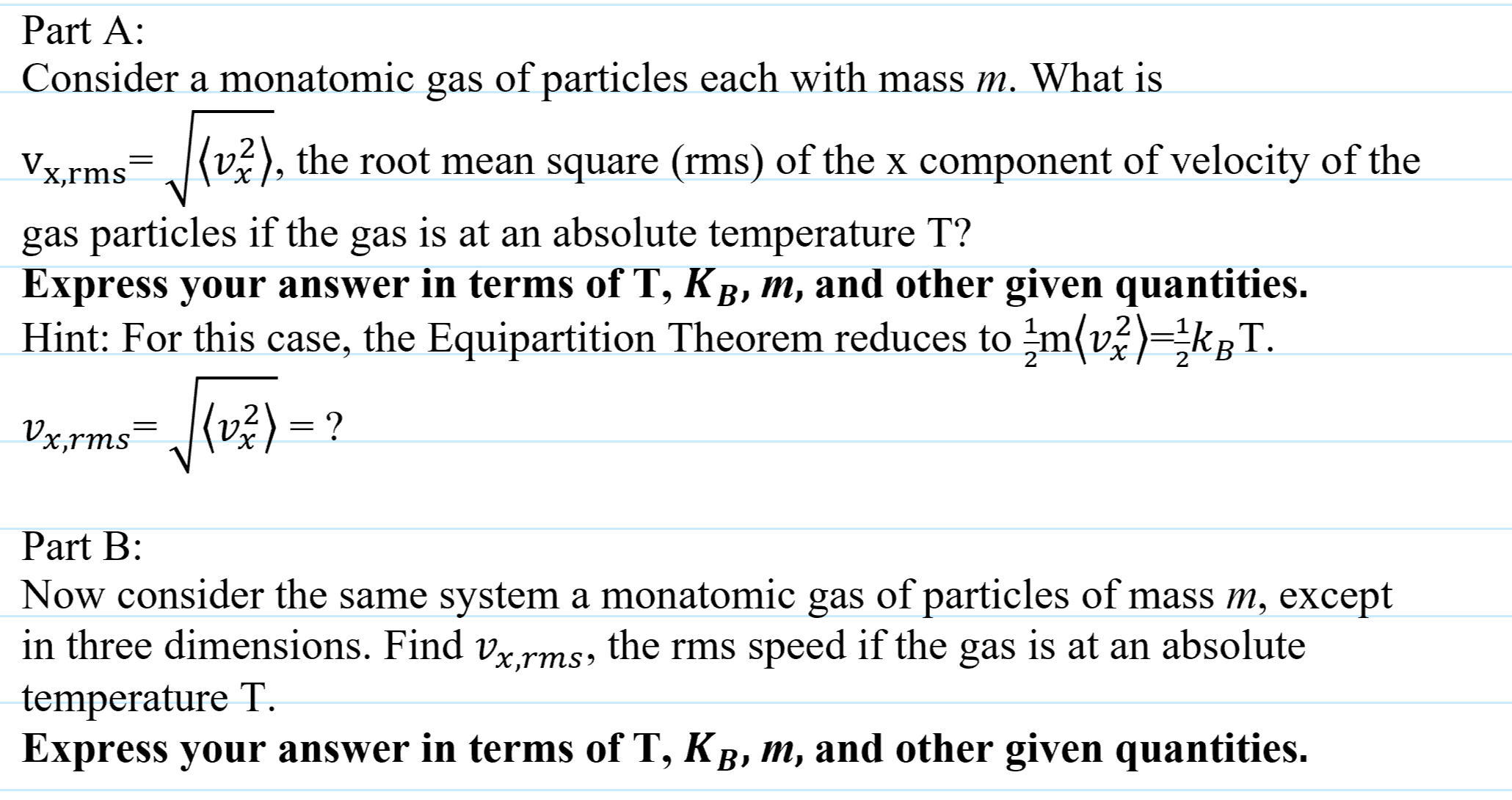
SOLVED: As a sample of argon gas is heated in a sealed container; its root- mean-square speed changes from 350 m/s to 550 m/s . The mass of an argon atom is 6.63